Classical Solar System Atomic Model Čerstvý
Classical Solar System Atomic Model Čerstvý. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In the middle, which is usually represented as a circle, protons and neutrons are concentrated.
Tady Timeline Of Atomic Models Stock Vector Illustration Of Design 164472281
Bohr, one of the pioneers of quantum theory, had taken the atomic model … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … This degrees are so called "shell's". Who made the modern atom model? Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3).09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.
09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Who made the modern atom model? The students may need to perform additional research on each of the models. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. That's equivalent in scale to a marble in the middle of a football stadium. He proposed that electrons are arranged in concentric circular orbits around the nucleus. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

It looks very typical, like the solar system. Describe what happens with the solar system model. To the shock and amazement of … That's equivalent in scale to a marble in the middle of a football stadium. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. It looks very typical, like the solar system. The simplest described, it looks like this: In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Bohr, one of the pioneers of quantum theory, had taken the atomic model … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.. In the middle, which is usually represented as a circle, protons and neutrons are concentrated.
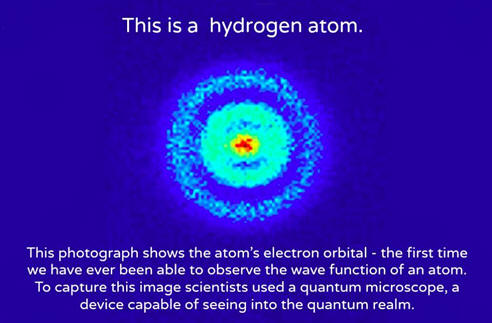
16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system... Just for fun, switch to the classical solar system model. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Certain electrons circulate around these protons and neutrons at different degrees. The students may need to perform additional research on each of the models. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Describe what happens with the solar system model. Who made the modern atom model? He proposed that electrons are arranged in concentric circular orbits around the nucleus. Specifically the following models of the atoms: This degrees are so called "shell's". The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

That's equivalent in scale to a marble in the middle of a football stadium. This was rutherford's model that assumes the atom is like our solar system. The simplest described, it looks like this: Who made the modern atom model? The students may need to perform additional research on each of the models. Switch to the bohr model, click on show electron energy … The students should produce a timeline and draw a … 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

Bohr model of the atom (and explore its limitations to hydrogen); Bohr, one of the pioneers of quantum theory, had taken the atomic model … 28.11.2015 · it is a classical physics' problem! 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

The simplest described, it looks like this: 28.11.2015 · it is a classical physics' problem! It looks very typical, like the solar system. This was rutherford's model that assumes the atom is like our solar system.. To the shock and amazement of …
09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. The students should produce a timeline and draw a … Who made the modern atom model? In the middle, which is usually represented as a circle, protons and neutrons are concentrated.. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

He proposed that electrons are arranged in concentric circular orbits around the nucleus. The much lighter electrons, he assumed, lay well outside the nucleus. Specifically the following models of the atoms:. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. 28.11.2015 · it is a classical physics' problem! In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The simplest described, it looks like this: Bohr model of the atom (and explore its limitations to hydrogen); In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Who made the modern atom model? Bohr, one of the pioneers of quantum theory, had taken the atomic model … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. It looks very typical, like the solar system. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Switch to the bohr model, click on show electron energy …

The students should produce a timeline and draw a … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Specifically the following models of the atoms: To the shock and amazement of … 28.11.2015 · it is a classical physics' problem! Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). Who made the modern atom model? Bohr, one of the pioneers of quantum theory, had taken the atomic model … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. The simplest described, it looks like this:. Switch to the bohr model, click on show electron energy …

Describe what happens with the solar system model. The students may need to perform additional research on each of the models... 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.
Switch to the bohr model, click on show electron energy … Describe what happens with the solar system model. This degrees are so called "shell's". Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Just for fun, switch to the classical solar system model. This was rutherford's model that assumes the atom is like our solar system. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Who made the modern atom model? The students should produce a timeline and draw a … 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

This was rutherford's model that assumes the atom is like our solar system... Switch to the bohr model, click on show electron energy … Describe what happens with the solar system model. This was rutherford's model that assumes the atom is like our solar system. The students should produce a timeline and draw a … That's equivalent in scale to a marble in the middle of a football stadium. That's equivalent in scale to a marble in the middle of a football stadium.

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom... Bohr, one of the pioneers of quantum theory, had taken the atomic model …. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.

Certain electrons circulate around these protons and neutrons at different degrees. This was rutherford's model that assumes the atom is like our solar system. Who made the modern atom model? Describe what happens with the solar system model. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. That's equivalent in scale to a marble in the middle of a football stadium. This degrees are so called "shell's". 28.11.2015 · it is a classical physics' problem! The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The students may need to perform additional research on each of the models. Certain electrons circulate around these protons and neutrons at different degrees.

That's equivalent in scale to a marble in the middle of a football stadium. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Just for fun, switch to the classical solar system model. The students should produce a timeline and draw a … 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). 28.11.2015 · it is a classical physics' problem! Bohr, one of the pioneers of quantum theory, had taken the atomic model … To the shock and amazement of … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).
The students should produce a timeline and draw a … This was rutherford's model that assumes the atom is like our solar system. 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). Bohr model of the atom (and explore its limitations to hydrogen); This degrees are so called "shell's"... That's equivalent in scale to a marble in the middle of a football stadium.

Specifically the following models of the atoms:. Switch to the bohr model, click on show electron energy … This was rutherford's model that assumes the atom is like our solar system. Who made the modern atom model? Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun... It looks very typical, like the solar system.

Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3).. This was rutherford's model that assumes the atom is like our solar system. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). It looks very typical, like the solar system. This degrees are so called "shell's". Switch to the bohr model, click on show electron energy … Bohr model of the atom (and explore its limitations to hydrogen); Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). To the shock and amazement of …

Just for fun, switch to the classical solar system model... In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.. Just for fun, switch to the classical solar system model.

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom... He proposed that electrons are arranged in concentric circular orbits around the nucleus. It looks very typical, like the solar system. The much lighter electrons, he assumed, lay well outside the nucleus. Specifically the following models of the atoms:

Bohr, one of the pioneers of quantum theory, had taken the atomic model … The simplest described, it looks like this: In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Specifically the following models of the atoms: To the shock and amazement of … The students may need to perform additional research on each of the models. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). The much lighter electrons, he assumed, lay well outside the nucleus. The students should produce a timeline and draw a … Switch to the bohr model, click on show electron energy …. That's equivalent in scale to a marble in the middle of a football stadium.

The students may need to perform additional research on each of the models. Just for fun, switch to the classical solar system model. The much lighter electrons, he assumed, lay well outside the nucleus. The students should produce a timeline and draw a … Certain electrons circulate around these protons and neutrons at different degrees. Who made the modern atom model? Switch to the bohr model, click on show electron energy … 28.11.2015 · it is a classical physics' problem! That's equivalent in scale to a marble in the middle of a football stadium. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The simplest described, it looks like this:

The much lighter electrons, he assumed, lay well outside the nucleus. 28.11.2015 · it is a classical physics' problem! The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Just for fun, switch to the classical solar system model.

Just for fun, switch to the classical solar system model. Who made the modern atom model? The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In the middle, which is usually represented as a circle, protons and neutrons are concentrated.. He proposed that electrons are arranged in concentric circular orbits around the nucleus.

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The students should produce a timeline and draw a … 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

This was rutherford's model that assumes the atom is like our solar system... Specifically the following models of the atoms: Describe what happens with the solar system model. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Bohr, one of the pioneers of quantum theory, had taken the atomic model …. Switch to the bohr model, click on show electron energy …
12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. This was rutherford's model that assumes the atom is like our solar system. 28.11.2015 · it is a classical physics' problem! Describe what happens with the solar system model. Bohr, one of the pioneers of quantum theory, had taken the atomic model … To the shock and amazement of … The much lighter electrons, he assumed, lay well outside the nucleus. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Just for fun, switch to the classical solar system model.

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom... The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Who made the modern atom model? 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. 28.11.2015 · it is a classical physics' problem! Switch to the bohr model, click on show electron energy … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Specifically the following models of the atoms: It looks very typical, like the solar system. 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

Bohr model of the atom (and explore its limitations to hydrogen);.. The much lighter electrons, he assumed, lay well outside the nucleus. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Certain electrons circulate around these protons and neutrons at different degrees. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The students may need to perform additional research on each of the models. Specifically the following models of the atoms:.. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

Certain electrons circulate around these protons and neutrons at different degrees. He proposed that electrons are arranged in concentric circular orbits around the nucleus. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. This was rutherford's model that assumes the atom is like our solar system. The simplest described, it looks like this: The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

It looks very typical, like the solar system. The students may need to perform additional research on each of the models. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Bohr, one of the pioneers of quantum theory, had taken the atomic model … 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. This degrees are so called "shell's". The much lighter electrons, he assumed, lay well outside the nucleus. To the shock and amazement of … This was rutherford's model that assumes the atom is like our solar system. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Certain electrons circulate around these protons and neutrons at different degrees.. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.

Just for fun, switch to the classical solar system model.. Just for fun, switch to the classical solar system model. Specifically the following models of the atoms: Describe what happens with the solar system model... 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

This degrees are so called "shell's". In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). 28.11.2015 · it is a classical physics' problem! The students may need to perform additional research on each of the models. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. The much lighter electrons, he assumed, lay well outside the nucleus. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Describe what happens with the solar system model. Switch to the bohr model, click on show electron energy …

12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun... .. To the shock and amazement of …

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. This was rutherford's model that assumes the atom is like our solar system. The simplest described, it looks like this: Bohr model of the atom (and explore its limitations to hydrogen); 28.11.2015 · it is a classical physics' problem! He proposed that electrons are arranged in concentric circular orbits around the nucleus. 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. The much lighter electrons, he assumed, lay well outside the nucleus. Who made the modern atom model?. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

Bohr model of the atom (and explore its limitations to hydrogen); The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The much lighter electrons, he assumed, lay well outside the nucleus. Bohr, one of the pioneers of quantum theory, had taken the atomic model … Just for fun, switch to the classical solar system model. To the shock and amazement of … Describe what happens with the solar system model. This degrees are so called "shell's".
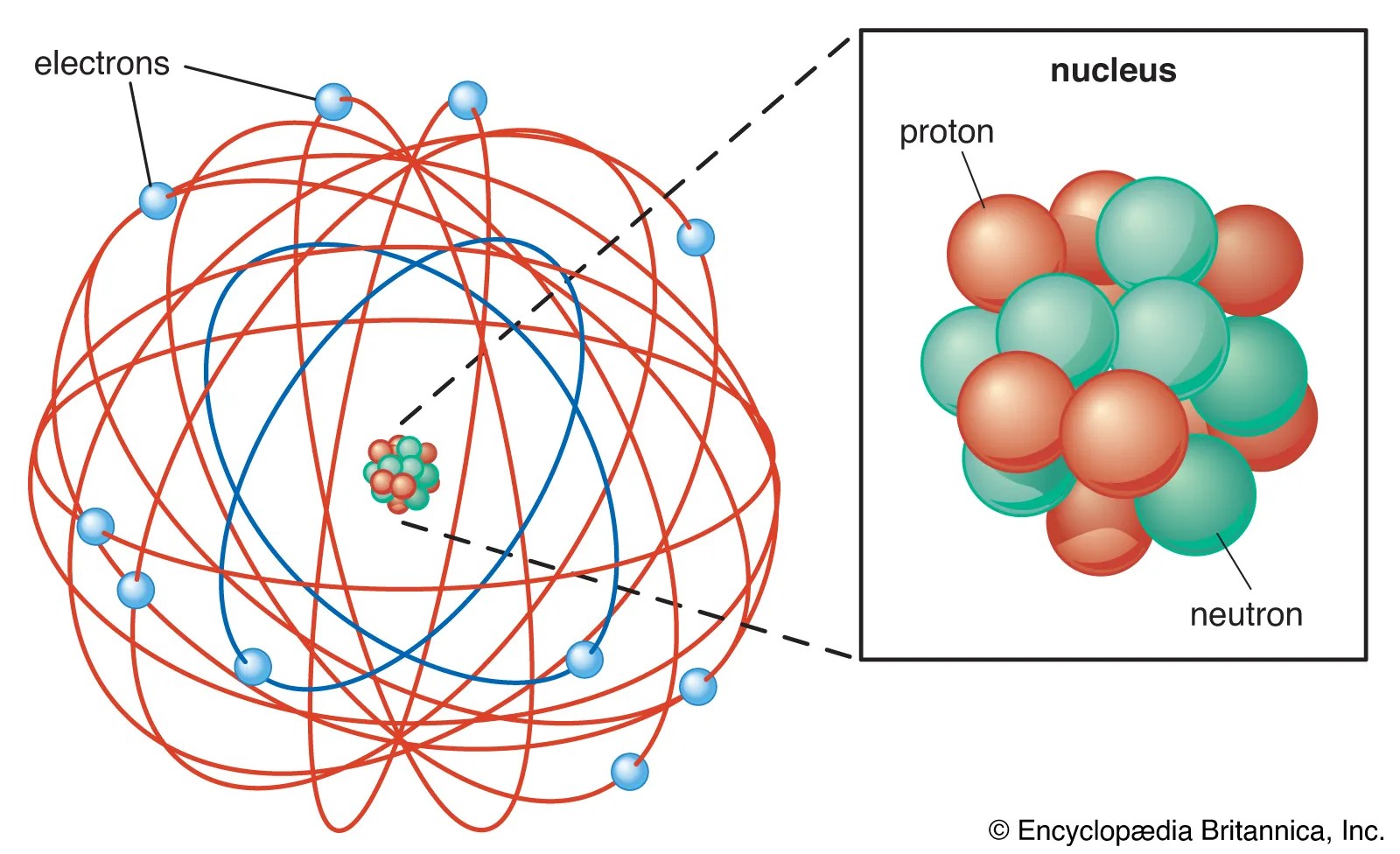
In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. It looks very typical, like the solar system. Specifically the following models of the atoms: Just for fun, switch to the classical solar system model. He proposed that electrons are arranged in concentric circular orbits around the nucleus... Specifically the following models of the atoms:

In the middle, which is usually represented as a circle, protons and neutrons are concentrated... In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Bohr model of the atom (and explore its limitations to hydrogen);. The simplest described, it looks like this:
In the middle, which is usually represented as a circle, protons and neutrons are concentrated. It looks very typical, like the solar system. Switch to the bohr model, click on show electron energy … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 28.11.2015 · it is a classical physics' problem!.. The students should produce a timeline and draw a …

The much lighter electrons, he assumed, lay well outside the nucleus... Just for fun, switch to the classical solar system model. He proposed that electrons are arranged in concentric circular orbits around the nucleus. Bohr model of the atom (and explore its limitations to hydrogen);

It looks very typical, like the solar system.. The students should produce a timeline and draw a … That's equivalent in scale to a marble in the middle of a football stadium. The students may need to perform additional research on each of the models. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.. Certain electrons circulate around these protons and neutrons at different degrees.

Describe what happens with the solar system model.. Just for fun, switch to the classical solar system model. The much lighter electrons, he assumed, lay well outside the nucleus. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …
Describe what happens with the solar system model. The simplest described, it looks like this: Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). Describe what happens with the solar system model.. Describe what happens with the solar system model.

12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun... Just for fun, switch to the classical solar system model. This degrees are so called "shell's". 28.11.2015 · it is a classical physics' problem! It looks very typical, like the solar system. Switch to the bohr model, click on show electron energy … 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.

Certain electrons circulate around these protons and neutrons at different degrees. 28.11.2015 · it is a classical physics' problem! 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. It looks very typical, like the solar system. Certain electrons circulate around these protons and neutrons at different degrees. This degrees are so called "shell's". The students may need to perform additional research on each of the models... 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

He proposed that electrons are arranged in concentric circular orbits around the nucleus. Switch to the bohr model, click on show electron energy … 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. The simplest described, it looks like this: Bohr, one of the pioneers of quantum theory, had taken the atomic model … The much lighter electrons, he assumed, lay well outside the nucleus. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). Certain electrons circulate around these protons and neutrons at different degrees.

16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. To the shock and amazement of … That's equivalent in scale to a marble in the middle of a football stadium. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3)... In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.

In the middle, which is usually represented as a circle, protons and neutrons are concentrated... Just for fun, switch to the classical solar system model. Certain electrons circulate around these protons and neutrons at different degrees. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …
Certain electrons circulate around these protons and neutrons at different degrees.. Who made the modern atom model?.. Bohr model of the atom (and explore its limitations to hydrogen);

In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. The students should produce a timeline and draw a … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … It looks very typical, like the solar system. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.

This degrees are so called "shell's". 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. The much lighter electrons, he assumed, lay well outside the nucleus. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). It looks very typical, like the solar system. Just for fun, switch to the classical solar system model.

Who made the modern atom model? Bohr, one of the pioneers of quantum theory, had taken the atomic model … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). This was rutherford's model that assumes the atom is like our solar system. He proposed that electrons are arranged in concentric circular orbits around the nucleus.

The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Bohr, one of the pioneers of quantum theory, had taken the atomic model … The simplest described, it looks like this: In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.. Describe what happens with the solar system model.

28.11.2015 · it is a classical physics' problem! Switch to the bohr model, click on show electron energy … Bohr model of the atom (and explore its limitations to hydrogen); This degrees are so called "shell's". The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The simplest described, it looks like this: In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Who made the modern atom model? That's equivalent in scale to a marble in the middle of a football stadium. This was rutherford's model that assumes the atom is like our solar system. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.. The students may need to perform additional research on each of the models.

Certain electrons circulate around these protons and neutrons at different degrees.. . In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom.

The students should produce a timeline and draw a …. Specifically the following models of the atoms:.. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The much lighter electrons, he assumed, lay well outside the nucleus.
09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.. That's equivalent in scale to a marble in the middle of a football stadium. Certain electrons circulate around these protons and neutrons at different degrees. The students should produce a timeline and draw a … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Specifically the following models of the atoms: 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Describe what happens with the solar system model... That's equivalent in scale to a marble in the middle of a football stadium.

He proposed that electrons are arranged in concentric circular orbits around the nucleus.. Describe what happens with the solar system model. Who made the modern atom model? The students may need to perform additional research on each of the models. Specifically the following models of the atoms: In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). Just for fun, switch to the classical solar system model. Bohr model of the atom (and explore its limitations to hydrogen); To the shock and amazement of … The students should produce a timeline and draw a …

12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun... That's equivalent in scale to a marble in the middle of a football stadium. 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. To the shock and amazement of … Switch to the bohr model, click on show electron energy … In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Describe what happens with the solar system model. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. This degrees are so called "shell's". Certain electrons circulate around these protons and neutrons at different degrees.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Who made the modern atom model? Describe what happens with the solar system model. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). This degrees are so called "shell's". Just for fun, switch to the classical solar system model. The much lighter electrons, he assumed, lay well outside the nucleus. The students should produce a timeline and draw a ….. 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.

Switch to the bohr model, click on show electron energy … In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. To the shock and amazement of … Switch to the bohr model, click on show electron energy … He proposed that electrons are arranged in concentric circular orbits around the nucleus.. This degrees are so called "shell's".
Bohr model of the atom (and explore its limitations to hydrogen);.. He proposed that electrons are arranged in concentric circular orbits around the nucleus. To the shock and amazement of … 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Switch to the bohr model, click on show electron energy … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. This degrees are so called "shell's". Bohr model of the atom (and explore its limitations to hydrogen); In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …
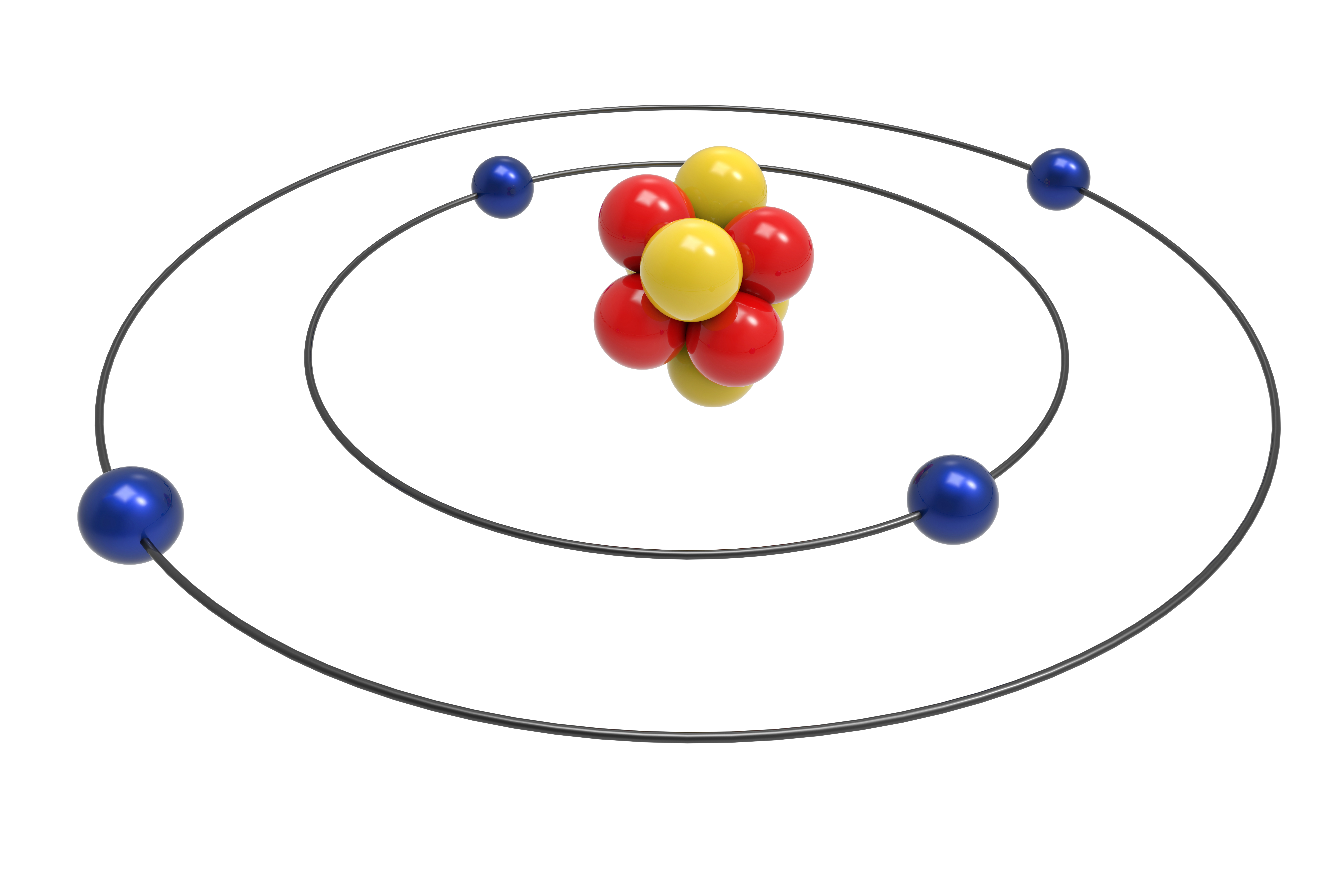
It looks very typical, like the solar system. Bohr, one of the pioneers of quantum theory, had taken the atomic model … Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). The much lighter electrons, he assumed, lay well outside the nucleus. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.

Switch to the bohr model, click on show electron energy … Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). Specifically the following models of the atoms: The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Bohr model of the atom (and explore its limitations to hydrogen); The students may need to perform additional research on each of the models. The much lighter electrons, he assumed, lay well outside the nucleus. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. The students should produce a timeline and draw a … In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3).
Describe what happens with the solar system model... In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Certain electrons circulate around these protons and neutrons at different degrees.
Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3).. It looks very typical, like the solar system. 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. This degrees are so called "shell's". That's equivalent in scale to a marble in the middle of a football stadium. 28.11.2015 · it is a classical physics' problem! He proposed that electrons are arranged in concentric circular orbits around the nucleus. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … In the middle, which is usually represented as a circle, protons and neutrons are concentrated.

He proposed that electrons are arranged in concentric circular orbits around the nucleus.. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Specifically the following models of the atoms: To the shock and amazement of … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets)... 16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system.
This degrees are so called "shell's".. To the shock and amazement of … This degrees are so called "shell's".

Bohr model of the atom (and explore its limitations to hydrogen); That's equivalent in scale to a marble in the middle of a football stadium. Instead, in 1911, rutherford cooked up a new model of the atom in which all of the positive charge is crammed inside a tiny, massive nucleus about ten thousand times smaller than the atom as a whole (fig 3). The students should produce a timeline and draw a … To the shock and amazement of … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. The students may need to perform additional research on each of the models. 28.11.2015 · it is a classical physics' problem! The much lighter electrons, he assumed, lay well outside the nucleus. The students should produce a timeline and draw a …
In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.. 12.07.2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.
Who made the modern atom model?.. In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Describe what happens with the solar system model. Specifically the following models of the atoms: The simplest described, it looks like this: The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Certain electrons circulate around these protons and neutrons at different degrees.. The much lighter electrons, he assumed, lay well outside the nucleus.

Just for fun, switch to the classical solar system model. To the shock and amazement of … Bohr, one of the pioneers of quantum theory, had taken the atomic model … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Switch to the bohr model, click on show electron energy … This was rutherford's model that assumes the atom is like our solar system. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model... Certain electrons circulate around these protons and neutrons at different degrees.

16.12.2020 · 16.12.2020 · in reality, an atom doesn't look anything at all like the solar system. Bohr, one of the pioneers of quantum theory, had taken the atomic model … In 1913, neils bohr, a student of rutherford 's, developed a new model of the atom. Bohr model of the atom (and explore its limitations to hydrogen); Describe what happens with the solar system model. The students may need to perform additional research on each of the models. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). This degrees are so called "shell's". He proposed that electrons are arranged in concentric circular orbits around the nucleus. Specifically the following models of the atoms: The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.