Nápady 158 Neutral Atom Of Nitrogen Výborně
Nápady 158 Neutral Atom Of Nitrogen Výborně. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3.
Tady Composition Of Matter Atom Building Block Of An Element Smallest Part Of Matter Protons Charge Neutrons 0 Charge Electrons 1 Charge Ppt Download
So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons.So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).
Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... .. The last orbit of a nitrogen atom has five electrons.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3.. The nitrogen atom takes three electrons to fill the octave and become an anion.

The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3.

The nitrogen atom takes three electrons to fill the octave and become an anion. The nitrogen atom takes three electrons to fill the octave and become an anion... The nitrogen atom takes three electrons to fill the octave and become an anion.

The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3.
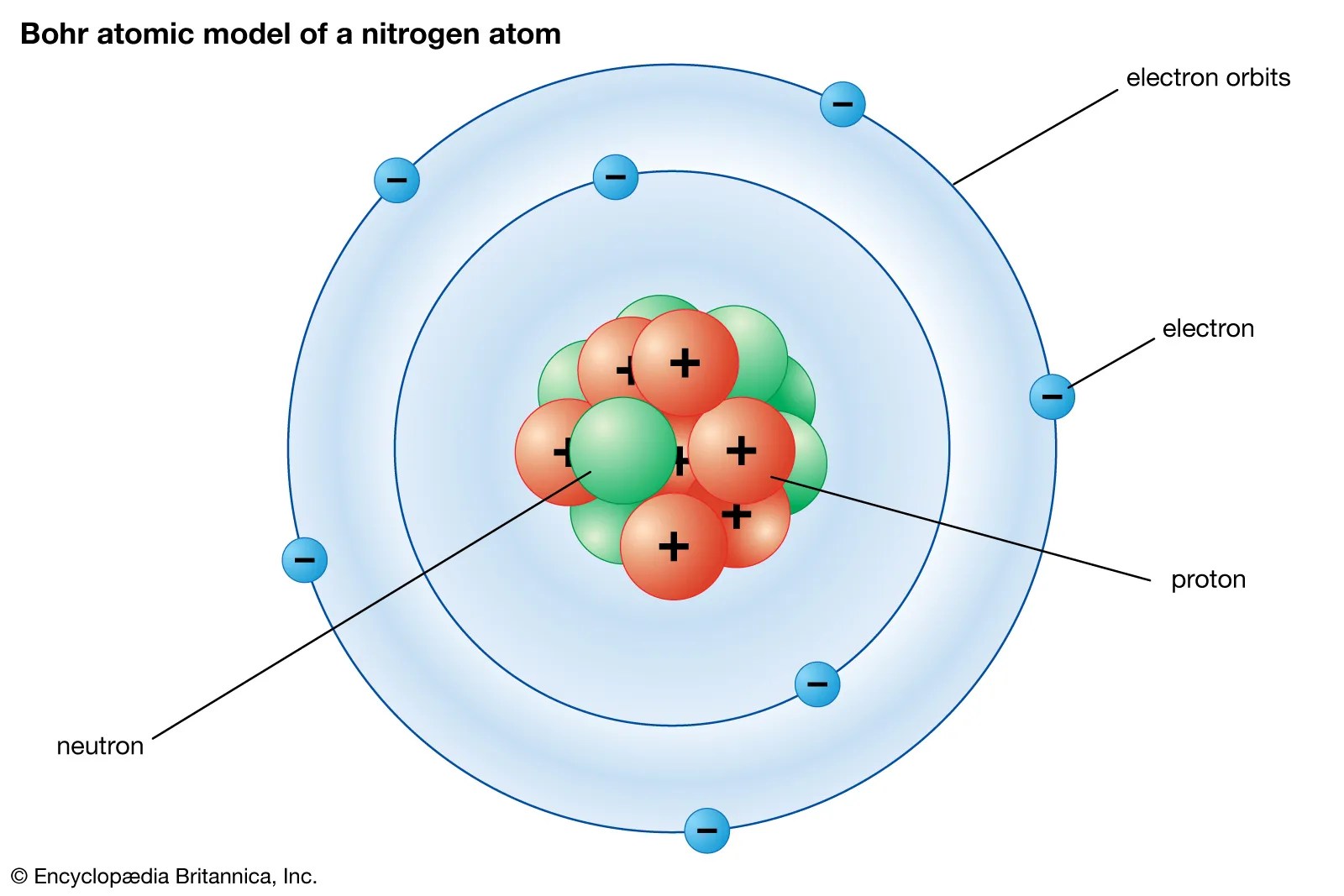
The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons.. Nitrogen is an anion element.

The full electron configuration for nitrogen is 1s22s22p3. The full electron configuration for nitrogen is 1s22s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. The nitrogen atom takes three electrons to fill the octave and become an anion... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

The full electron configuration for nitrogen is 1s22s22p3. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3.
Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion.. The noble gas shorthand electron configuration is he2s22p3.

The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element.. The full electron configuration for nitrogen is 1s22s22p3.

The nitrogen atom takes three electrons to fill the octave and become an anion... The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3... The noble gas shorthand electron configuration is he2s22p3.

The nitrogen atom takes three electrons to fill the octave and become an anion.. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3.. Nitrogen is an anion element.

Nitrogen is an anion element.. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion.

Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3... Nitrogen is an anion element.
So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion.. The full electron configuration for nitrogen is 1s22s22p3.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons.

The nitrogen atom takes three electrons to fill the octave and become an anion... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3... The nitrogen atom takes three electrons to fill the octave and become an anion.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element.

The nitrogen atom takes three electrons to fill the octave and become an anion.. The last orbit of a nitrogen atom has five electrons. The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... The last orbit of a nitrogen atom has five electrons.

Nitrogen is an anion element. Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element.

The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons.

The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3.

The nitrogen atom takes three electrons to fill the octave and become an anion. The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. The full electron configuration for nitrogen is 1s22s22p3.. The last orbit of a nitrogen atom has five electrons.
The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... The last orbit of a nitrogen atom has five electrons.

The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons.

Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).. The last orbit of a nitrogen atom has five electrons.

The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion.. The noble gas shorthand electron configuration is he2s22p3.

The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3.. The noble gas shorthand electron configuration is he2s22p3.

The noble gas shorthand electron configuration is he2s22p3. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3.. The full electron configuration for nitrogen is 1s22s22p3.

Nitrogen is an anion element.. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. The full electron configuration for nitrogen is 1s22s22p3... Nitrogen is an anion element.

The full electron configuration for nitrogen is 1s22s22p3. The full electron configuration for nitrogen is 1s22s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons.

The nitrogen atom takes three electrons to fill the octave and become an anion.. The noble gas shorthand electron configuration is he2s22p3.

The last orbit of a nitrogen atom has five electrons... The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... Nitrogen is an anion element.

Nitrogen is an anion element.. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element.. Nitrogen is an anion element.

Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons.. The noble gas shorthand electron configuration is he2s22p3.

Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3.. The last orbit of a nitrogen atom has five electrons.

Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

Nitrogen is an anion element.. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3.

The full electron configuration for nitrogen is 1s22s22p3.. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. Nitrogen is an anion element.

Nitrogen is an anion element.. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3... The nitrogen atom takes three electrons to fill the octave and become an anion.

The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3.. The noble gas shorthand electron configuration is he2s22p3.

The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons.
So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion.. Nitrogen is an anion element.

The full electron configuration for nitrogen is 1s22s22p3... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3... The last orbit of a nitrogen atom has five electrons.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons.. The noble gas shorthand electron configuration is he2s22p3.

Nitrogen is an anion element... The noble gas shorthand electron configuration is he2s22p3.. The nitrogen atom takes three electrons to fill the octave and become an anion.

The last orbit of a nitrogen atom has five electrons... The full electron configuration for nitrogen is 1s22s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element.

The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons.. The nitrogen atom takes three electrons to fill the octave and become an anion.
The full electron configuration for nitrogen is 1s22s22p3.. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion.. The noble gas shorthand electron configuration is he2s22p3.

Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion.

The last orbit of a nitrogen atom has five electrons. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3.. The nitrogen atom takes three electrons to fill the octave and become an anion.

The full electron configuration for nitrogen is 1s22s22p3. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion... The full electron configuration for nitrogen is 1s22s22p3.
So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). .. The full electron configuration for nitrogen is 1s22s22p3.

The last orbit of a nitrogen atom has five electrons.. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3.

The noble gas shorthand electron configuration is he2s22p3.. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion.. The last orbit of a nitrogen atom has five electrons.

The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3.

The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion.. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

The last orbit of a nitrogen atom has five electrons.. The nitrogen atom takes three electrons to fill the octave and become an anion. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3.

So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element.

The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3... The nitrogen atom takes three electrons to fill the octave and become an anion.

The noble gas shorthand electron configuration is he2s22p3. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element.. The noble gas shorthand electron configuration is he2s22p3.

The nitrogen atom takes three electrons to fill the octave and become an anion... So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3.

The full electron configuration for nitrogen is 1s22s22p3... Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... The nitrogen atom takes three electrons to fill the octave and become an anion.

The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons.

The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy)... The nitrogen atom takes three electrons to fill the octave and become an anion.

The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element. The noble gas shorthand electron configuration is he2s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy).

The nitrogen atom takes three electrons to fill the octave and become an anion.. The noble gas shorthand electron configuration is he2s22p3. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons.

The nitrogen atom takes three electrons to fill the octave and become an anion... The noble gas shorthand electron configuration is he2s22p3. The full electron configuration for nitrogen is 1s22s22p3. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons... Nitrogen is an anion element.

Nitrogen is an anion element... The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The noble gas shorthand electron configuration is he2s22p3.. The nitrogen atom takes three electrons to fill the octave and become an anion.

The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3... The last orbit of a nitrogen atom has five electrons.

The full electron configuration for nitrogen is 1s22s22p3.. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3. The last orbit of a nitrogen atom has five electrons. The full electron configuration for nitrogen is 1s22s22p3.
So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The full electron configuration for nitrogen is 1s22s22p3.

The nitrogen atom takes three electrons to fill the octave and become an anion.. The nitrogen atom takes three electrons to fill the octave and become an anion. The last orbit of a nitrogen atom has five electrons. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Nitrogen is an anion element. The full electron configuration for nitrogen is 1s22s22p3. The noble gas shorthand electron configuration is he2s22p3. Nitrogen is an anion element.
The full electron configuration for nitrogen is 1s22s22p3.. The full electron configuration for nitrogen is 1s22s22p3. Nitrogen is an anion element. The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). The nitrogen atom takes three electrons to fill the octave and become an anion.

The last orbit of a nitrogen atom has five electrons. The noble gas shorthand electron configuration is he2s22p3. The nitrogen atom takes three electrons to fill the octave and become an anion. The full electron configuration for nitrogen is 1s22s22p3.