Seznamy Atom Mass Number
Seznamy Atom Mass Number. Protons and neutrons are collectively known as nucleons. Thus, the atomic mass of an atom is almost the same as its mass number. The sum of the mass number and the atomic number for an atom … In other words, it is the sum of the number of nucleons in an atom. The mass number of an atom is its total number of protons and neutrons.
Tady Chem 101 Lecture 2
In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. An electron is almost negligible in weight. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. It is a decimal number.20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).
20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Protons and neutrons are collectively known as nucleons. The mass number of an atom is its total number of protons and neutrons. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. It is a decimal number. An electron is almost negligible in weight. For other isotopes, the isotopic mass … The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.. An electron is almost negligible in weight. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The mass number of an atom is its total number of protons and neutrons. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Protons and neutrons are collectively known as nucleons. It is a whole number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element... For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.

It is a whole number. Mass number is often denoted using a capital letter a. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons... The mass number is determined by the number of protons and neutrons combined.

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.. It is a decimal number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is its total number of protons and neutrons.. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a decimal number. For other isotopes, the isotopic mass … The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. It is a whole number.. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

The mass number is determined by the number of protons and neutrons combined. For other isotopes, the isotopic mass …. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. Mass number is often denoted using a capital letter a. It is a whole number. For other isotopes, the isotopic mass …

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Thus, the atomic mass of an atom is almost the same as its mass number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. Protons and neutrons are collectively known as nucleons. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a decimal number. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is its total number of protons and neutrons. An electron is almost negligible in weight. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For other isotopes, the isotopic mass … It is a decimal number. Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same. Protons and neutrons are collectively known as nucleons. The mass number is determined by the number of protons and neutrons combined. The mass number is determined by the number of protons and neutrons combined.

20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
The mass number of an atom is its total number of protons and neutrons. An electron is almost negligible in weight. The mass number of an atom is its total number of protons and neutrons.

In other words, it is the sum of the number of nucleons in an atom. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Protons and neutrons are collectively known as nucleons.. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.

The mass number is determined by the number of protons and neutrons combined.. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). For other isotopes, the isotopic mass …

It is a decimal number. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. It is a decimal number. The sum of the mass number and the atomic number for an atom … In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atoms of different elements usually have different mass numbers , but they can be the same. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom.. It is a whole number.

For other isotopes, the isotopic mass ….. The sum of the mass number and the atomic number for an atom … It is a whole number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. The mass number of an atom is its total number of protons and neutrons. The mass number is determined by the number of protons and neutrons combined. It is a decimal number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element... The mass number of an atom is its total number of protons and neutrons.

Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The sum of the mass number and the atomic number for an atom … Thus, the atomic mass of an atom is almost the same as its mass number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Mass number is often denoted using a capital letter a. It is a whole number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. The mass number is determined by the number of protons and neutrons combined. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. An electron is almost negligible in weight.. Mass number is often denoted using a capital letter a.

04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. An electron is almost negligible in weight.

Mass number is often denoted using a capital letter a. The sum of the mass number and the atomic number for an atom … Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The mass number of an atom is its total number of protons and neutrons. Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. In other words, it is the sum of the number of nucleons in an atom. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. It is a whole number.. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
Protons and neutrons are collectively known as nucleons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. An electron is almost negligible in weight. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom.. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.

Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a whole number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Thus, the atomic mass of an atom is almost the same as its mass number. The mass number is determined by the number of protons and neutrons combined... It is a decimal number.
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. Thus, the atomic mass of an atom is almost the same as its mass number. An electron is almost negligible in weight. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. In other words, it is the sum of the number of nucleons in an atom. For other isotopes, the isotopic mass … It is a whole number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Protons and neutrons are collectively known as nucleons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. For other isotopes, the isotopic mass …

It is a decimal number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. Thus, the atomic mass of an atom is almost the same as its mass number. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). The mass number is determined by the number of protons and neutrons combined. It is a decimal number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. The mass number of an atom is its total number of protons and neutrons. Mass number is often denoted using a capital letter a. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus.

Mass number is often denoted using a capital letter a... 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. An electron is almost negligible in weight. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a whole number. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). Protons and neutrons are collectively known as nucleons. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom.

Thus, the atomic mass of an atom is almost the same as its mass number.. For other isotopes, the isotopic mass … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. It is a decimal number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. The sum of the mass number and the atomic number for an atom … 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu).. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is its total number of protons and neutrons. Thus, the atomic mass of an atom is almost the same as its mass number. Mass number is often denoted using a capital letter a. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. It is a whole number.

Mass number is often denoted using a capital letter a. It is a whole number. The mass number is determined by the number of protons and neutrons combined. In other words, it is the sum of the number of nucleons in an atom.

For other isotopes, the isotopic mass … Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom.. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

An electron is almost negligible in weight. . Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons... 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.. Mass number is often denoted using a capital letter a.
For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.. Protons and neutrons are collectively known as nucleons. Thus, the atomic mass of an atom is almost the same as its mass number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. It is a decimal number. The mass number is determined by the number of protons and neutrons combined. In other words, it is the sum of the number of nucleons in an atom. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.
14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom.

The sum of the mass number and the atomic number for an atom …. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. An electron is almost negligible in weight.. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
For other isotopes, the isotopic mass …. The sum of the mass number and the atomic number for an atom … 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. Thus, the atomic mass of an atom is almost the same as its mass number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... The sum of the mass number and the atomic number for an atom …

04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same.. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.

11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Atoms of different elements usually have different mass numbers , but they can be the same. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. An electron is almost negligible in weight. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). In other words, it is the sum of the number of nucleons in an atom.. An electron is almost negligible in weight.

Thus, the atomic mass of an atom is almost the same as its mass number... It is a decimal number. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. For other isotopes, the isotopic mass …. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus.

The mass number of an atom is its total number of protons and neutrons. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a whole number. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. It is a decimal number. The mass number is determined by the number of protons and neutrons combined.. Thus, the atomic mass of an atom is almost the same as its mass number.

The mass number of an atom is its total number of protons and neutrons... 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). For other isotopes, the isotopic mass … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. For other isotopes, the isotopic mass … Thus, the atomic mass of an atom is almost the same as its mass number. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.
The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). It is a whole number. For other isotopes, the isotopic mass … An electron is almost negligible in weight. It is a decimal number. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol... Mass number is often denoted using a capital letter a. It is a whole number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Thus, the atomic mass of an atom is almost the same as its mass number. For other isotopes, the isotopic mass … 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

Mass number is often denoted using a capital letter a. Thus, the atomic mass of an atom is almost the same as its mass number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number of an atom is its total number of protons and neutrons. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Mass number is often denoted using a capital letter a. Atoms of different elements usually have different mass numbers , but they can be the same. In other words, it is the sum of the number of nucleons in an atom. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The sum of the mass number and the atomic number for an atom … The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

An electron is almost negligible in weight. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). An electron is almost negligible in weight. The mass number of an atom is its total number of protons and neutrons. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Protons and neutrons are collectively known as nucleons. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu).

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For other isotopes, the isotopic mass … For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. The mass number is determined by the number of protons and neutrons combined. The sum of the mass number and the atomic number for an atom … The mass number of an atom is its total number of protons and neutrons. Thus, the atomic mass of an atom is almost the same as its mass number... The mass number is determined by the number of protons and neutrons combined.

The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. Thus, the atomic mass of an atom is almost the same as its mass number. The mass number of an atom is its total number of protons and neutrons.

Protons and neutrons are collectively known as nucleons. The sum of the mass number and the atomic number for an atom … 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Protons and neutrons are collectively known as nucleons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number is determined by the number of protons and neutrons combined. Mass number is often denoted using a capital letter a. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu).. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus.
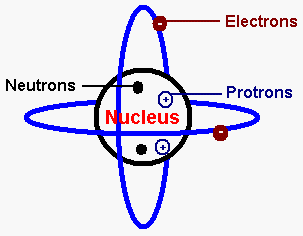
It is a decimal number.. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Thus, the atomic mass of an atom is almost the same as its mass number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. For other isotopes, the isotopic mass … The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Thus, the atomic mass of an atom is almost the same as its mass number. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons... 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The mass number is determined by the number of protons and neutrons combined... 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom …. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. The mass number of an atom is its total number of protons and neutrons. Thus, the atomic mass of an atom is almost the same as its mass number. For other isotopes, the isotopic mass … Atoms of different elements usually have different mass numbers , but they can be the same. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). It is a decimal number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined.. The sum of the mass number and the atomic number for an atom …

The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. An electron is almost negligible in weight. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a whole number. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element... For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.

An electron is almost negligible in weight... .. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol... For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.

04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom... 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For other isotopes, the isotopic mass … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

For other isotopes, the isotopic mass … . Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.

The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Thus, the atomic mass of an atom is almost the same as its mass number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). An electron is almost negligible in weight. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. In other words, it is the sum of the number of nucleons in an atom. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. It is a whole number.. Thus, the atomic mass of an atom is almost the same as its mass number.

The mass number is determined by the number of protons and neutrons combined.. The sum of the mass number and the atomic number for an atom … It is a whole number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. An electron is almost negligible in weight. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. It is a decimal number. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. The mass number of an atom is its total number of protons and neutrons.

Thus, the atomic mass of an atom is almost the same as its mass number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). For other isotopes, the isotopic mass … Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a decimal number. 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. An electron is almost negligible in weight. Protons and neutrons are collectively known as nucleons.. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. In other words, it is the sum of the number of nucleons in an atom. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Protons and neutrons are collectively known as nucleons. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. For other isotopes, the isotopic mass … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The sum of the mass number and the atomic number for an atom … An electron is almost negligible in weight. The mass number is determined by the number of protons and neutrons combined.

Protons and neutrons are collectively known as nucleons. Atoms of different elements usually have different mass numbers , but they can be the same. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu).. For other isotopes, the isotopic mass …

An electron is almost negligible in weight... Thus, the atomic mass of an atom is almost the same as its mass number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number is determined by the number of protons and neutrons combined. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. The mass number of an atom is its total number of protons and neutrons. Protons and neutrons are collectively known as nucleons. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.. 21.11.2020 · the unit of measure for mass is the atomic mass unit (amu).
One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Protons and neutrons are collectively known as nucleons. The sum of the mass number and the atomic number for an atom … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The mass number is determined by the number of protons and neutrons combined. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Mass number is often denoted using a capital letter a.. Mass number is often denoted using a capital letter a.

11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. It is a decimal number. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is often denoted using a capital letter a. 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom.

The sum of the mass number and the atomic number for an atom ….. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom … It is a decimal number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. Atoms of different elements usually have different mass numbers , but they can be the same.. Mass number is often denoted using a capital letter a.

One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. It is a decimal number. In other words, it is the sum of the number of nucleons in an atom. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. It is a whole number.

04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom... The sum of the mass number and the atomic number for an atom … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. The mass number is determined by the number of protons and neutrons combined. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

Thus, the atomic mass of an atom is almost the same as its mass number... The sum of the mass number and the atomic number for an atom … 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. The mass number is determined by the number of protons and neutrons combined. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. An electron is almost negligible in weight.. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it.

An electron is almost negligible in weight. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number.

An electron is almost negligible in weight. The mass number of an atom is its total number of protons and neutrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons.

The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. The mass number is determined by the number of protons and neutrons combined. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 04.02.2020 · the mass number is the sum of the number of protons and neutrons in an atom. For other isotopes, the isotopic mass … 20.09.2021 · the number of protons that a chemical element has in its centre (nucleus) is called the atomic number. Thus, the atomic mass of an atom is almost the same as its mass number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. Protons and neutrons are collectively known as nucleons.. An electron is almost negligible in weight.

21.11.2020 · the unit of measure for mass is the atomic mass unit (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from it. The mass number is determined by the number of protons and neutrons combined. Thus, the atomic mass of an atom is almost the same as its mass number. 11.10.2021 · an atom's mass number, also called atomic mass number or nucleon number, is the total number of protons and neutrons present in an atomic nucleus. It is a decimal number. It is a whole number. 14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements.

14.12.2008 · mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol... 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).
